
1 Institute of Management, Nirma University, Ahmedabad, Gujrat, India
Creative Commons Non Commercial CC BY-NC: This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 License (http://www.creativecommons.org/licenses/by-nc/4.0/) which permits non-Commercial use, reproduction and distribution of the work without further permission provided the original work is attributed.
In the context of the ongoing COVID-19 pandemic, the Indian medical innovation ecosystem confronted substantial challenges in meeting the pressing healthcare requirements of the nation. These challenges demand a comprehensive examination to discern the critical issues in Indian Medical Sociology about medical innovations. The primary objective of this study is to assess the intricate interplay between the approaches employed in drug and vaccine development and their consequential effects on affordability and accessibility. Concurrently, it aims to evaluate the efficacy of the existing policy measures affecting social divisions that have emerged because the supply-driven health system has exacerbated.
A qualitative research methodology has been adopted for this inquiry, utilising thematic analysis to dissect the intricacies of the Indian medical innovation ecosystem. The study unfurls the following noteworthy discoveries that contribute to a nuanced understanding of the landscape: (a) it becomes evident that the predominant focus within the Indian medical innovation ecosystem revolves around creating affordable medical technologies and pharmaceuticals, often overshadowing the pursuit of cutting-edge advancements. (b) The strategies employed in developing drugs and vaccines exhibit a conspicuous inclination towards products that promise higher profits, subsequently giving rise to issues associated with affordability and accessibility. (c) While policy measures have been implemented with the intent of addressing the concern of affordability, their actual efficacy in ensuring equitable access to medical innovations raises significant doubts. (d) A discernible imbalance emerges between India's industrial and public health priorities, with economic growth overshadowing the imperative of bridging the existing social disparities.
These insights collectively underline the imperative for strategic policy interventions, a more balanced approach to development, and establishing a multi-institutional framework. These actions are essential to confront the challenges related to affordability, accessibility and the aggravated social divisions that persist within the ecosystem. The study brings out the indispensable importance of cultivating a socially responsible and all-encompassing medical innovation ecosystem, not only as a means to address the prevailing healthcare challenges but also to foster an environment that guarantees equitable access to transformative medical innovations for all segments of society.
Medical sociology, innovation, public health, medical innovation ecosystem, public policy, access to healthcare
Introduction
The COVID-19 pandemic in India has introduced unprecedented disease prevention and management challenges. This has given rise to a multitude of obstacles in the field of medical sociology, as well as the broader healthcare industry’s innovation ecosystem. Throughout history, the occurrence and resurgence of contagious diseases have posed significant concerns for developing and underdeveloped nations worldwide. Various political and economic conditions have hindered these countries’ growth and attention towards medical advancements. A noteworthy example is the inadequate focus on diseases, such as ‘Ebola’, ‘Dengue’ and ‘Japanese encephalitis’ in India and Nepal, as well as ‘Influenza’ and ‘Malaria’ in numerous African and South Asian nations. Vaccines, drugs and medical devices for these diseases have not received attention. This disparity is evident in global instances of ‘Severe Acute Respiratory Syndrome’ (SARS) in 2002 and the ‘Zika’ virus outbreak in 2016. Diseases of this nature in impoverished nations have either been neglected or not considered priorities for development. The COVID-19 pandemic has laid bare the ill-preparedness of the world in addressing contagious disease issues, transcending the dichotomy of rich and poor.
Consequently, this situation provides a fascinating avenue for sociological exploration of the threat posed by such diseases in global societies, considering that the perception of infection risk has permeated all socioeconomic strata. Such investigations into medical sociology can be grounded in the epistemology of the contemporary organisation of innovation ecosystems. Current medical innovation systems have been shaped by the values and ethos of techno-capitalism, exerting substantial influence over governments’ policy priorities. Thus, it becomes imperative to comprehend the distinctions between public health policies and medical innovations in India and the world.
Over the past four decades, there has been a remarkable progression in medical advancements, particularly in pharmaceuticals, vaccinations and medical equipment. This progress and the interplay and innovations within disciplines such as biotechnology, biomedicine, bioinformatics, genomics and synthetic biology have fueled this progress and have given rise to a new breed of competitive markets and intellectual property protection regimes fostered by collaborations between governments, academic and entrepreneurial research institutions and industries on a global scale. A consequential result of these transformative shifts has been the proliferation of Research and Development (R&D) endeavours within the healthcare domain. Private enterprises primarily drive this upsurge, thanks partly to the emergence of venture capital. However, amidst these advancements, there has been a significant fall in government funding allocated to public health initiatives. This decrease in public health funding, except for a few diseases addressed by state health departments, university divisions and specific research institutes, raises essential considerations.
Globally recognised is the significant contribution of the medical innovation ecosystem to disease control, treatment and management. Laal (2012) delineates a spectrum of innovative medicines, encompassing ‘aspirin’, ‘insulin’, ‘oral contraceptives’, ‘penicillin’, ‘smart pills’, ‘statins’, ‘vaccines’ and ‘viagra’. Similarly, a range of technologies, including ‘electrocardiography’, ‘electronic health records’, ‘laser surgery’, ‘magnetic resonance imaging (MRI)’, ‘nano-healing’, ‘organ transplant’, ‘ultrasound’, ‘X-ray’, etc. have emerged as pivotal medical innovations in recent decades. Also, notable medical devices such as ‘artificial hearts’, ‘artificial joints’, ‘bone injector drills’, ‘dialysis machines’, ‘handheld medical scanners’, ‘lens implants’, ‘robotic catheters’, ‘skin antennas’, etc. exemplify the diverse landscape of medical innovation. Discoveries of the human genome allowed medical sciences to explore enormous possibilities that expand medical innovation’s scope regarding personalised medicines, prognosis and diagnosis of high-risk diseases. Similarly, stem cell research, cancer therapies, combinational drug therapies and DNA-sequencing technologies are innovations joining the league of medical innovations that contributed to the development of the healthcare industry and had a long-lasting impact on human society.
These research and innovations have contributed to the development of drugs, vaccines and medical devices through academic-industrial collaborations. An increase in thrust to such collaborations is observed in India, but rigorous commercialisation and monopolisation of innovations from such collaborations pose vital questions for public health. It poses sociological questions about such collaborations’ power dynamics, authority and political economy. For instance, who decides priorities for medical innovation? What significant contemplations require looking into the disease or components of public health of any beneficiaries? Are these collaborations, R&D and innovations adding value to society, or are they just meant to satisfy the needs of techno-capitalism? Do such innovations lead to vulnerabilities, marginality or social inequalities? Will such devices, drugs or medical innovations lead to medicalisation? If so, the burden will be borne by whom? And many more.
The biggest challenge experienced in India during the COVID-19 pandemic in the medical innovation ecosystem is achieving a balanced approach for drug and vaccine development, controlling price and affordability. The study of existing literature suggests that such challenges must be addressed at the policy, management and administration levels. It is a known truth that returns drive R&D projects in the Indian pharma industry, as drug and vaccine development were financially costly projects with the lowest success rate. The therapeutic trajectory of drug and vaccine development predominantly revolves around high-yield products and target demographics. Nonetheless, existing literature does not fully acknowledge the social and emotional toll incurred in mitigating disease burdens, thus complicating the defense of arguments advocating for increased financial allocation toward future disease mitigation efforts. Research and Development expenditure often correlates with the projected costs of new pharmaceutical products. While various policy interventions implemented at different stages of the pandemic have ostensibly addressed the affordability of vaccines and drugs, they have not uniformly ensured enhanced access. Furthermore, certain policy measures aimed at alleviating the financial burden of R&D expenditure have fallen short of guaranteeing affordability. For instance, initiatives such as reducing or standardising value-added tax on medications, cutting or eliminating excise duties, commitments to establishing biotech parks, providing financial aid to start-ups, lifting financial caps on Foreign Direct Investments (FDIs) and fostering collaboration between academia and industry have incentivised R&D in medical innovation. However, its translation in affordability and accessibility of drugs or vaccines is still questionable.
Another challenge observed during the COVID-19 pandemic in India is about balancing Industrial (or capitalist?) and public health priorities. India’s public health and drug policies are still exclusive frameworks in terms of their objectives and functions, with multiple overlapping concerns (George et al., 2018). For instance, in India, the Ministry of Chemicals and Fertilizers oversees drugs and pharmaceuticals, whereas in developed nations, drug development falls under the purview of the Health Ministry. Consequently, industrial expansion, regulatory measures and promotional initiatives related to FDIs, exports and tax incentives within the medical sector, including pharmaceutical and biopharmaceutical enterprises, are predominantly influenced by industrial and export-import economic policies rather than health-focussed social policies (Figure 1). Because of such a scenario, more thrust is observed on investment promotion and industrial or economic growth rather than focussing on bridging the social divide between the medical innovation ecosystem and society.
There exists a scarce amount of scientific literature discussing over social costs and benefits of medical innovations in India. In India, supply-driven healthcare produces results in a variety of societal divides like (a) it emasculates policy focus on the epidemiological burden of poor geographically and social sections of society; (b) the benefits of medical innovations are disturbed by and for economically better-off populations while the burden of cost in terms of clinical trials generally borne by the poverty-ridden population. In this vital context, it is urgently required to fix policy priorities for the drug industry to have formal social obligations, directing funds for more affordable cutting-edge R&D and translating these R&D investments into the public welfare of the heterogeneous population of the country through a multi-institutional framework.
Figure 1. Regulatory Framework Observed During the COVID-19 Pandemic When India Sought Vaccines and Other Medical Innovations.
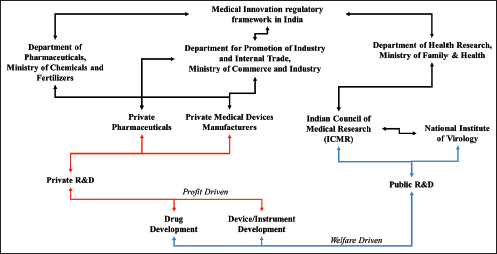
It becomes crucial to address vital research gaps that are observed in existing scientific literature and thus, the paper contributes towards grasping (a) policy measures adopted in India to address issues related to access and affordability of medical innovations in India during COVID-19 pandemic conditions; and (b) contribution of supply-driven health systems towards social divisions leading to unequal access to medical innovations.
Based on the literature survey, the research questions were formulated which is used in the next section of the paper. The following section of the article focusses on details about the study, research questions and objectives. The paper’s third section comprises a qualitative analysis of the Indian medical innovation ecosystem. This analysis encompasses various thematic dimensions, including examining the roles played by different stakeholders, the emphasis on the development of affordable technologies and drugs, the challenges associated with achieving a balanced development approach and the impact of industrial and public health priorities. Interpretations are supported by incorporating pertinent literature and critical observations.
Lastly, the concluding section effectively summarises the key insights derived from the analysis and critical observations. It highlights challenges confronting the medical innovation ecosystem in India, explicitly focussing on affordability, accessibility and the need to bridge the social divide. The study underscores the necessity for policy interventions and establishing a multi-institutional framework to address these challenges effectively.
Previous Works (Literature Review)
Table 1 provides an overview of selected studies that have contributed to understanding the medical innovation ecosystem. Each study focusses on theoretical frameworks related to a variety of areas. These studies cumulatively contribute to the body of scientific knowledge and inform the analysis presented in the current research work.
Table 1. Major Studies Reviewed.
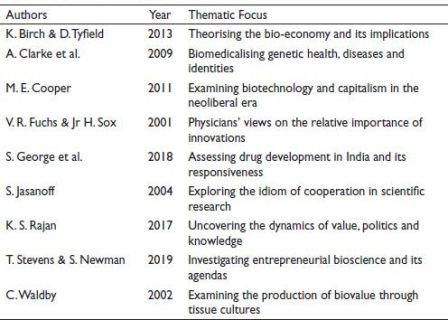
Fuchs and Sox (2001) conducted a noteworthy study categorising 30 significant innovations in America. Among these innovations, angiotensin-converting enzyme, balloon angioplasty, inhibitors, mammography MRI and computed tomographic scanning and statins, emerged as the five most impactful on human society. Conversely, innovations such as bone marrow transplant, calcium channel blockers, conscious sedation, non-sedating antihistamines, sildenafil (Viagra), were identified as the least essential. Such categorisation of innovations raises questions because the study was conducted based on the experiences and perceptions of participants. Such studies do not satisfy questions related to the relevance of innovations to public health. Instead of judging the usefulness of innovations, one should judge innovations based on their impact or contribution to public health. Along with innovations in the medical field, medical sociology also needs to grasp conditions of social changes created by pharmaceutical and biopharmaceutical innovations that have attracted a significant amount of attention from scholars worldwide for various reasons. The studies discussed illustrate the involvement of medical sociologies in navigating epistemological shifts, examining political economy dynamics, exploring collaborative production processes and analysing impacts and consequences for access, affordability and the medicalisation of the human body and entire human society (Birch & Tyfeld, 2013; Cooper, 2011; Pandey et al., 2014; Rose, 2007; Waldby, 2002).
The commodification of science on others has influenced public health policies heavily. The commodification of science has thus impacted the research ecosystem where research and development are viewed as business Levins & Lewontin (1985). This scenario is evident from growing literature in the area of medical sociology (mostly on biopolitics and patient subjectification) and the political economy inclined towards medical innovations and biomedical technology.
The concept of biovalue, as introduced by Waldby (2002), serves to elucidate the intricate nexus between biotechnology, life sciences and capitalism. This theoretical framework draws upon Marxian theories of value and Foucauldian notions of biopolitics. Biopolitics, as conceptualised by Michel Foucault, focusses on the governance and management of populations through the regulation of their biological and social life. In the medical innovation ecosystem, biopolitics presents how power operates within the healthcare system, shaping the development and distribution of medical technologies, drugs and vaccines. One aspect where biopolitics is pertinent is understanding the prioritisation and allocation of resources within the medical innovation ecosystem. Such perceptions allow us to grasp the challenges of balancing industrial and public health priorities, where economic growth and profit-driven motives often take precedence over equitable access to healthcare innovations. This alignment with biopolitics can be observed in the tension between industrial growth, economic policies and the social obligations of the medical industry towards the larger population. The concept of biopolitics allows for a critical examination of how power relations, economic interests and political forces influence the direction and outcomes of medical innovation, potentially leading to inequalities and a social divide.
Such conceptual framework is further extended by various scholars like Rajan (2006), who illustrated how the novel know-how became the basis of medical innovations that boosted the advancements within the domain of biomedical research and how new technology emerged from such innovations pave the way for the development of a techno-capitalistic paradigm of the healthcare industry as well as affect public health policies. Birch and Tyfield (2013) presented different perceptions. The authors did subscribe to biovalue, bio capital and biopower concepts but not wholly different from the value of labour within a Marxist framework. Their central argument is that value is a mere representation of knowledge value that can be produced, reproduced, exchanged and circulated through various processes and institutions.
Despite such disagreement on ‘bioconcepts’, the scholars also acknowledge the power of the (bio) knowledge economy and its impact on the social deterministic model of health and well-being to biomedical reductionism and individualised care.
Interestingly, some studies attempt to apprehend the epistemic changes observed in the techno-capitalistic biomedical paradigm. These studies heavily focus on the nature of capital and its strategies of circulation, co-production, accumulation and subjectification of patients (Birch & Tyfeld, 2013; Clarke et al., 2009; Cooper, 2011; Jasanoff, 2004; Rajan, 2017; Rose, 2007; Stevens & Newman, 2019; Strasser, 2014; ). Rose (2007) elaborated epistemologies of the ‘molecular self’, which views the body as a collective of various organs and cells grasped as separate entities for research. He epistemologically approaches biomedicine and claims it is biomedical reductionism, which presents a shift from ‘corporeal self’ to ‘molecular self’. Such conceptions open an avenue for a wide range of medical innovations focused on customising bodily appearance, redefining normalcy, medicalising the body, etc. Clarke et al. (2009) focussed on techno-scientific innovations organised around biomedicines that led to the bio-medicalisation of society. The authors explained that biomedicalisation is a process that is consolidating, hegemonised and legitimised by the State. Increased health surveillance in the biomedical paradigm presents a shift in the healthcare approach, that is, treatment of risk, which further commodifies health, well-being and lifestyles.
About Study
The study adopts a qualitative approach, presents thematic propositions and leads to a hypothesis for further research. Thematic propositions, such as the influence of the social divide on the affordability and accessibility of drugs and vaccines, provide a foundation for further exploration. It emphasises the importance of multi-institutional collaboration to translate R&D investments into public welfare for the diverse population. The need to balance industrial growth and public health priorities is underscored, suggesting the alignment of drug policies with social needs rather than solely economic considerations. Ethical concerns regarding distributing benefits and burdens in drug development are also highlighted. The paper also suggests a hypothesis highlighting the financial burden on future disease mitigations resulting from prioritising high-profit products and target groups in drug and vaccine development. It recommends policy interventions to address affordability and accessibility issues, such as price regulation and targeted subsidies.
Research Questions
Research Objectives
The overarching objectives of this study are fourfold––firstly, to meticulously identify and analyse the multifaceted challenges that the Indian medical innovation ecosystem encountered during the COVID-19 pandemic. Secondly, to conduct a comprehensive examination of the intricate interplay between various drug and vaccine development strategies and their direct influence on the crucial aspects of affordability and accessibility within the context of India. Thirdly, to undertake a rigorous evaluation of the efficacy and impact of the prevailing policy measures that were implemented to address the intricacies of affordability and accessibility concerning medical innovations amidst the pandemic’s turbulence. Lastly, a vital goal is to critically assess the pronounced societal divisions that have emerged as a result of the supply-driven healthcare system, delving into their far-reaching implications about the populace’s access to pivotal medical innovations.
Medical Innovation Ecosystem of India During the COVID-19 Pandemic
In the context of COVID-19 in India, it is changes in both pharmaceutical and biopharmaceutical domains during the post-product patent period in India are observed. The Indian drug industry under new IPR realms observed consolidations through M&A (Mergers and Acquisitions), Out-Contracting, decentralising of production and interconnectedness with global value chains. The COVID-19 pandemic proved the powerful influence of biomedical and biopharmaceutical giants over public health policies. The surge in the expansion of research and collaboration networks, coupled with the active engagement of governments, research institutions, public sector laboratories, university departments, among others, is evident amidst the ongoing pandemic.
It is observed that despite an upsurge in income generation within the Indian pharmaceutical sector, the financial growth trajectory of multinational corporations has trailed behind that of Indian private enterprises. The private sector players in India boast substantial revenue and R&D expenditure, particularly within the realm of Indian biopharmaceuticals. Amidst the challenging landscape of the COVID-19 pandemic, the medical innovation ecosystems in India exhibit characteristics intricately intertwined with a multifaceted institutional framework, fostering the commercialisation, replication and realisation of specialised expertise. Such multifaceted institutional approach encompasses a diverse array of contributors, both domestic and international, including pharmaceutical and biopharmaceutical entities, contract organisations, research institutions, private healthcare facilities, as well as foreign and domestic venture capital investors. Moreover, state agencies such as DST, BIRAC, SAARC, etc. play pivotal roles alongside entities facilitating technology dissemination, funding and state-sponsored research bodies like the Council of Scientific and Industrial Research and the Indian Council of Medical Research. Further enriching this ecosystem are private and public research laboratories, individual and institutional entrepreneurs, bolstered by the support of media outlets, information service providers, civil society organisations, community-based volunteer groups and non-governmental organisations engaged in facilitating clinical trials, patient engagement initiatives and online pharmaceutical services. The network also extends to encompass health and wellness centers, various healthcare providers and ancillary service providers contributing indirectly to the ecosystem’s vitality. This entire gamut became part of the political economy driven by the marketing strategies of corporate giants. Such marketing strategies focussed on medical and social innovations that were further ‘used’ to determine and increase funding in R&D. These overwhelming politically driven fundraisings resulted in the development of products with improved investment returns. How much of these investments are translated into improving the population during a pandemic? It may have a hegemonic response.
In the domain of medical innovations ecosystems in India during the COVID-19 pandemic, a heavy focus was placed on R&D related to drugs/medicines, vaccines and medical devices. Most pharmaceuticals were inclined to branded generics, biosimilars and NCEs (new chemical entities), which was very low from 2000 to 2017. For instance, during the period spanning from 2000 to 2017, a total of 135 novel pharmaceuticals received approval for commercialisation. A substantial proportion, that is, 87% of these approved drugs were targeted towards addressing Non-communicable diseases (NCDs), while a mere 6% were designated for combating communicable diseases. Within these 6%, most drugs were meant for cancer, cardiovascular, dermatological, immunological, neurological and ophthalmological diseases (George, 2021).
Vaccine development in India during COVID-19 times has attracted not only intellectual attention but has also created a vast public hype. This domain attracted urgent focus from companies, the government and the public. Vaccine development in India’s biopharmaceutical corporate giants was previously limited to infectious diseases like chikungunya, hepatitis A, influenza, Japanese encephalitis, malaria, meningitis, pertussis, typhoid, etc.
The medical innovation ecosystem was more focused on NCDs, while most international companies focussed their R&D on developing high-end drugs and medical technologies for preventing and managing communicable diseases. Conversely, publicly funded institutes of the Indian medical innovation ecosystem are more focussed on creating affordable medical technologies and drugs. Only a few organisations (including start-ups) in the private sector can be observed as paying attention to developing cutting-edge technologies and drugs that can be marketed at affordable rates. The COVID-19 pandemic has proven that the social divide and challenges to addressing priorities in the innovation ecosystem are scarcely discussed in India.
Conclusion
The study has addressed the contemporary issues and fulfilled the objectives outlined. Firstly, it identified and analysed the challenges encountered by the Indian medical innovation ecosystem during the COVID-19 pandemic. By conducting a comprehensive analysis and incorporating relevant literature, the study presented the complex issues faced by the innovation ecosystem in the medical realm, such as the need for a balanced development approach, affordability concerns and social divisions.
Secondly, the study examined the relationship between drug and vaccine development approaches and their impact on affordability and accessibility in India. By analysing the R&D scenario of the Indian pharma industry and its focus on high-profit products, the study highlighted the implications for affordability and access to medical innovations. It addressed the financial burden of drug and vaccine development and its potential consequences for future disease mitigations.
Thirdly, the study evaluated the effectiveness of existing policy measures in addressing the affordability and accessibility of medical innovations during the pandemic. It discussed various policy measures implemented, such as reducing taxes, providing financial assistance to start-ups and linking academia with industry. The study critically examined the outcomes of these measures and their impact on ensuring affordability and accessibility, highlighting the limitations and areas for improvement.
Lastly, the study assessed the social divisions the supply-driven health system created and their implications for access to medical innovations. By emphasising the unequal distribution of benefits and burdens within the ecosystem, the study drew attention to the marginalised sections of society bearing the cost of clinical trials while the benefits primarily accrued to economically better-off populations. It underscored the need for policy priorities that promote formal social obligations, affordability, and accessibility to bridge the social divide.
Future directions will advance our understanding of the Indian medical innovation ecosystem and contribute to evidence-based policymaking. By leveraging complex methodologies and multidisciplinary approaches, researchers can unearth critical insights, propose effective strategies and foster a more inclusive and accessible healthcare system in India and beyond.
It becomes essential to explore the enduring ramifications of the pandemic on the medical innovation ecosystem and discern its impact on the priorities and strategies of stakeholders. By searching more deeply into the transformations in research and development investments, collaborations between academia and industry and the emergence of novel models for affordable and accessible medical innovations, researchers can gain comprehensive insights into the long-term effects of the pandemic. Understanding these effects will provide a foundation for future policy formulation and strategic decision-making in the healthcare sector.
A thorough evaluation of the policies implemented during the pandemic is warranted to gauge their effectiveness in addressing the affordability and accessibility of medical innovations. Such an evaluation can help identify gaps or limitations in the existing policies and offer recommendations for policy enhancements to ensure equitable access to healthcare technologies. This analysis allows researchers to contribute to evidence-based policymaking, empowering governments and healthcare authorities to optimise their strategies and interventions to benefit the wider population.
Conducting comparative analyses between the Indian medical innovation ecosystem and other countries or regions can illuminate best practices and successful models. Through in-depth case studies, researchers can explore the approaches that have effectively balanced industrial growth with public health priorities, achieved affordability in drug and vaccine development, or implemented policies that bridge social divides. This cross-country comparison will identify valuable lessons and facilitate knowledge exchange, fostering global collaboration to address the challenges medical innovation ecosystems face worldwide.
Gaining insights into the perspectives and experiences of various stakeholders within the medical innovation ecosystem is crucial. Exploring the viewpoints of healthcare professionals, industry leaders, policymakers and patients can provide a comprehensive understanding of the challenges and aspirations within the system. By integrating these perspectives, researchers can identify strategies to bridge the social divide, enhance affordability and improve access to medical innovations. This inclusive approach ensures that research outcomes align with the needs and aspirations of all stakeholders, resulting in more effective and sustainable solutions for healthcare challenges.
Lastly, investigating the adoption and diffusion of medical innovations in diverse socioeconomic contexts within India will yield valuable insights. This research can delve into the barriers and facilitators of technology uptake, identify strategies to overcome challenges and examine the impact of diffusion patterns on healthcare outcomes. Researchers can develop targeted interventions and policies to accelerate the widespread dissemination of medical innovations by unravelling the complexities of technology adoption and innovation diffusion. This, in turn, will contribute to narrowing the existing disparities in access to healthcare technologies and fostering equitable healthcare delivery across different population segments.
Recommendations
Adopting a Balanced Development Approach
The medical innovation ecosystem in India should expand the boundaries of medical advancements beyond profits. For this, it becomes imperative to balance breakthrough innovations, basic healthcare necessities and academia-industry-government collaborations. An incentivised (through tax exemptions, grants, etc.) collaborative research and development among (pharma and healthcare) industry, independent R&D units and government regulatory agencies will help to achieve a sustainable balanced development approach.
Affordability and Accessibility Concerns
A complex intertwined and intricate drug and vaccine development process holds centrality for medical innovations in India. A multifaceted approach is required at policy levels to alleviate the financial burden on the R&D of medical innovations. Such an approach should accompanied by socialist or welfare-inclined regulatory frameworks. The socialist or welfare-oriented regulatory frameworks should keep a check on the affordability and accessibility of medical innovations.
Pricing Models
A more holistic approach is required for scrutinising the pricing models of pharmaceutical products. Stricter implementation of pricing regulations would balance incentivising innovation and ensuring affordable access for all strata of society.
The Burden of Clinical Trial
The marginalised sections of the country withstanding the worst of clinical trial costs is a glaring social justice issue. This calls for a paradigm shift in policy priorities. Policies should be geared towards reducing the disparities in access to medical innovations. Incorporating strict formal social obligations into the healthcare system would ensure that the benefits of medical advancements are shared equitably across the population. This bridges the social divide and strengthens the overall fabric of healthcare accessibility.
Acknowledgements
The author is grateful to the anonymous referees of the journal for their extremely useful suggestions to improve the quality of the paper. Usual disclaimers apply.
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding
The authors received no financial support for the research, authorship and/or publication of this article.
ORCID iD
Pratham Parekh  https://orcid.org/0000-0002-6358-0677
https://orcid.org/0000-0002-6358-0677
Baid, C., & Baid, D. (2023). Funding failure: Determinants of persistence. IMIB Journal of Innovation and Management, 1(1), 58–64. https://doi.org/10.1177/ijim.221085417
Birch, K., & Tyfield, D. (2013). Theorising the bioeconomy: Biovalue, biocapital, bioeconomics or... what?. Science, Technology, & Human Values, 38(3), 299–327.
Clarke, A. E., J. Shim, S. Shostak & A. Nelson (2009). ‘Biomedicalising genetic health, diseases and identities’. In P. Atkinson P. Glasner Ma. Lock (Eds), Handbook of genetics and society: Mapping the new genomic era (pp. 21–40). Routledge.
Cooper, M. E. (2011). Life as surplus: Biotechnology and capitalism in the neoliberal era. University of Washington Press.
Faix, A. (2022). Qualitative innovation in the light of the normative: A minimal approach to promoting and measuring successful innovation in business. IMIB Journal of Innovation and Management, 1(1). https://doi.org/10.1177/ijim.221091004
Fuchs, V. R., &Sox Jr, H. C. (2001). Physicians’ views of the relative importance of thirty medical innovations. Health Affairs, 20(5), 30–42.
George, S. (2021). Medical innovation and disease burden: Conflicting priorities and the social divide in India. Cambridge University Press.
George, S., Chandran, A. B., Nadh, P. O., & Apurva, K. H. (2018). Is drug development in India responsive to the disease burden? Economic & Political Weekly, 53(30), 51.
Jasanoff, S. (Ed.). (2004). The idiom of cooperation. In States of knowledge: The co-production of science and social order (pp. 1–12). Routledge.
Laal, M. (2012). Innovation and medicine. Procedia Technology, 1, 469–473.
Levins, R., & Lewontin, R. (1985). In The dialectical biologist. Harvard University Press.
Lovaas, J. (2007). In The politics of life itself: Biomedicine, power, and subjectivity in the twenty-first century (p. 352). Nikolas Rose. Princeton University Press 2006. 15.95, ISBN 978-0-691-12191-8, paperback. Journal of Biosocial Science, 39(5), 795–796.
Pandey, A., Ploubidis, G. B., Clarke, L., & Dandona, L. (2018). Trends in catastrophic health expenditure in India: 1993 to 2014. In Bulletin of the World Health Organization (Vol. 96, p. 18). Princeton University Press.
Rajan, K. S. (2017). In Pharmocracy: Value, politics, and knowledge in global biomedicine. Duke University Press.
Rajan, S. (2002). Biocapital: The constitution of post-genomic life [Doctoral dissertation, Massachusetts Institute of Technology].
Rose, N. (2007). In The politics of life itself: Biomedicine, power, and subjectivity in the twenty-first century. Princeton University Press.
Stevens, T., & Newman, S. (2019). In Biotech juggernaut: Hope, hype, and hidden agendas of entrepreneurial bioscience. Routledge.
Strasser, B. J. (2014). Biomedicine: Meanings, assumptions, and possible futures. The Swiss Science and Innovation Council. https://www.swir.ch/images/stories/pdf/en/SWIR_1_2014_Biomedicine.pdf,.
Waldby, C. (2002). Stem cells, tissue cultures and the production of biovalue. Health, 6(3), 305–323.